Ethylene Glycol Is Used as an Automobile Antifreeze
Calculate how much the freezing point of water will be lowered. The addition of ethylene glycol to water raises the boiling point of the engine coolant and reduces the chances of a car s radiator boiling over.
Solved Ethylene Glycol Is Used As An Automobile Antifreeze And In The Manufacture Of Polyester Fibers The Name Glycol Stems From The Sweet Taste Of This Poisonous Compound Combustion Of 6 38 Mathrm Mg Of
Answer 1 of 4.

. Often colored fluorescent yellow-green when used in automotive antifreeze. Ethylene glycol is essentially nonvolatile and it does not dissociate in water. The name glycol stems from the sweet taste of this poisonous compound.
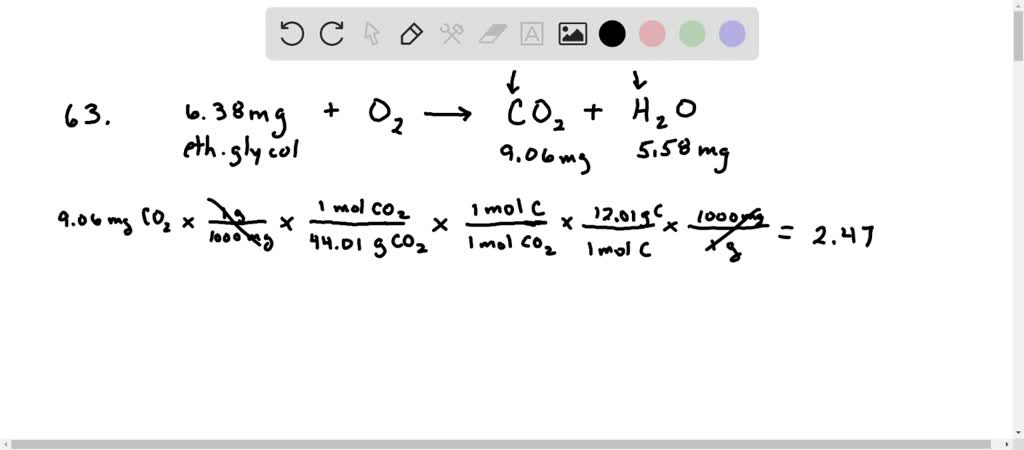
Ethylene glycol commonly used as automobile antifreeze has a density of 11088 gmL at room temperature. M6207 gmL and water d100 gmL at 20 degrees C. Combustion of 638 g of this compound gives 906 g of co2 and 558 g of h2o.
Part A What is the volume L of 100 kg of ethylene glycol. The name glycol stems from the sweet taste of this poisonous compound. It is clear colorless and practically odorless.
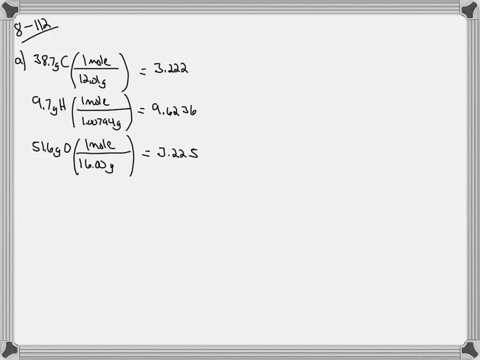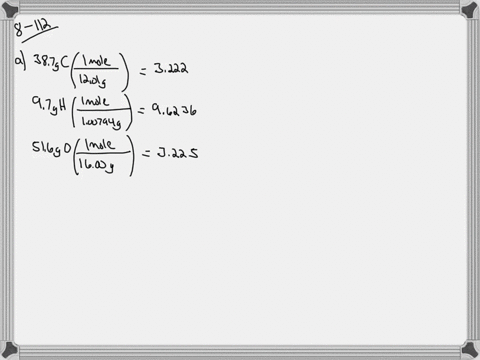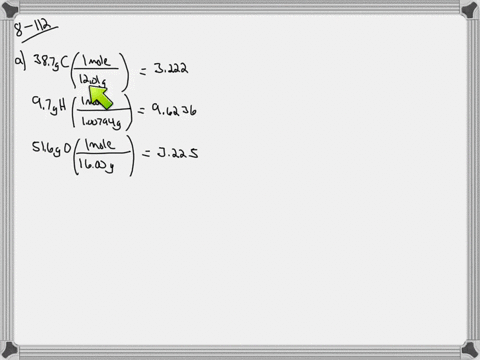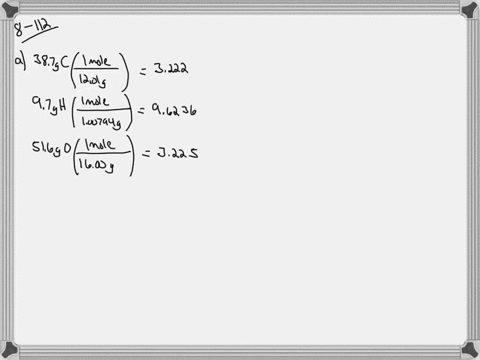
Ethylene glycol is used as an antifreeze agent. Ethylene glycol the substance used in automobile antifreeze is composed of 387 C 97 H and 516 O by mass. K f for water is 1.
If 400 g of ethylene glycol is added to 400 kg of water what is the molality. Examples include antifreeze hydraulic brake fluids some stamp pad inks ballpoint pens solvents paints plastics films and cosmetics. Ethylene glycol helps keep your cars engine from freezing in the winter and acts as a coolant to reduce overheating in the summer.
Of moles of O atoms 621 g 516 16 gmol 2 mol Hence molecular formula. Browse discover thousands of brands. It contains ethylene glycol a chemical which if ingested can cause damage to the.
Ethylene glycol C2H6O2 is used as an antifreeze in cars. DIYChemicals Ethylene Glycol Heat Transfer Fluid 100 Concentrate Technical Grade for Automotive Antifreeze Industrial Coolant Brake Fluid Solvents Cosmetics. Ethylene glycol is a chemical commonly used in many commercial and industrial applications including antifreeze and coolant.
Dont let the names trick you up. Automotive antifreeze consists of ethylene glycol C2H6O2 a nonvolatile electrolyte. In a cold climate water gets frozen causing damage to the radiators of a car.
The compound only contains c h and o. Of moles of H atoms 621 g 97 1 gmol 6 mol No. Ethylene glycol is a useful industrial compound found in many consumer products.
Calculate the boiling point and freezing point of a 250 mass percent of ethylene glycol in solution. Mono Ethylene Glycol or simply Ethylene Glycol is an organic compound having the molecular formula as CH2OHCH2OH. If the molecular mass of this compound is 124 amu what is the empirical and molecular formula.
8 5 K m o l 1 k g. Engine coolant is a mix of ethylene glycol and water. Ethylene glycol is used in automobile radiators as an antifreeze.
Coolant is antifreeze. The jugs you buy at the store are pre-diluted. American production cars did not begin using any antifreeze until 1923 when an ethylene oxide based coolant was first used.
Use the following information to determine the freezing point of an antifreeze solution made by mixing 25 L of ethylene glycol with 25 L of water. An automobile antifreeze mixture is made by mixing equal volumes of ethylene glycol d1114 gmL. This solution helps the car engine perform more efficiently and ensures that your car doesnt stop working in.
Ethylene glycol is an organic carbon based molecule most widely used as antifreeze in automobile engines and as an industrial solvent a chemical in which other substancesare dissolved. Ethylene glycol helps avoid the freezing of your cars engine in winter and serves as a coolant to minimize overheating in summer. Ethylene Glycol is widely used as an antifreeze and automotive heat transfer fluid.
By far this is the more toxic of the two types of antifreeze commonly sold today. 3 attempts remaining Part B What is the volume L of 200 lb of ethylene glycol. Antifreeze is toxic because it contains glycol a chemical that can be harmful to both humans and animals.
Therefore its always recommended to add ethylene glycol ie antifreeze to water in a 11 ratio and then pour this mixture into your cars radiator. Nearly all engines use a 11 ratio of coolant. One thing to bear in mind with modern coolants.
Inhibited ethylene glycol antifreeze mixes are available with additives that buffer the pH and reserve alkalinity of the solution to prevent oxidation of ethylene glycol and formation of these acids. Read customer reviews find best sellers. The compound contains only C.
The mass of ethylene glycol to be added to 4 kg water to prevent it from freezing at 6 o C. Ethylene Glycol the green antifreeze we have all been using as long as we can remember did not replace. In 1 mole of ethylene glycol.
There are two types of antifreeze and they differ in their toxicity. Ethylene glycol commonly used as automobile antifreeze has a specific gravity of 11088 at room temperature. Combustion of 638 mg of ethylene glycol gives 906 mg CO2 and 558 mg H2O.
Ethylene glycol is used as an automobile antifreeze and in the manufacture of polyester fibers. Ethylene glycol used in automobile antifreeze and in the production of polyester. Submit Previous Answers Request Answer Incorrect.
When ethylene glycol is used in a system it may become oxidized to five organic acids formic oxalic glycolic glyoxalic and acetic acid. Ethylene glycol is a chemical that is widely used in many commercial and industrial applications including antifreeze and refrigerants. Of moles of C atoms 621 g 387 12 gmol 2 mol No.
Then anything that you know can be important that I can learn from the process.
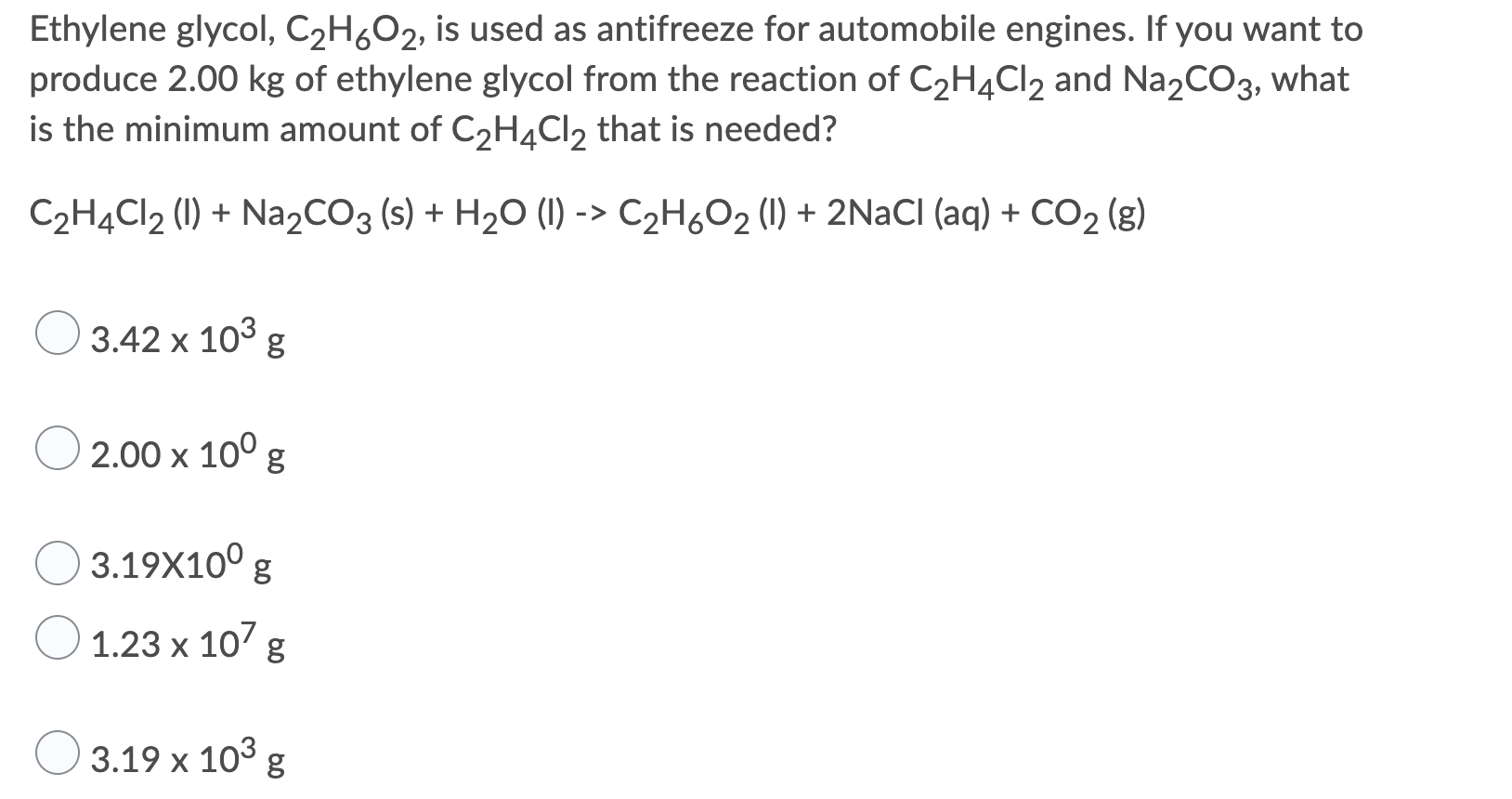
Solved Ethylene Glycol C2h602 Is Used As Antifreeze For Chegg Com

Solved Automobile Antifreeze Ethylene Glycol And Diethylene Glycol Are Used As Automobile Antifreeze And Are Produced By The Reactions Of Ethylene Oxide With Water As Follows A Mole Of Either Glycol In Water

Solved A Ethylene Glycol Eg Ch2ohch Oh Is An Organic Chegg Com

Why Is Ethylene Glycol Added To Water In Car Radiators Quora

Solved Ethylene Glycol Is Used As Antifreeze In Automobile Chegg Com

Ethylene Glycol Is Used As An Antifreeze In A Cold Cliamate Mass Of Ethylene Glycol Youtube
Ethylene Glycol Is Used As An Antifreeze In A Cold Climate Mass Of Ethylene Glycol Which Should Be Added To 4 Kg Of Water Sarthaks Econnect Largest Online Education Community
How To Extend The Life Of Your Engine Using Antifreeze And Coolants Witham News

Ethylene Glycol Ch2 Oh Ch2 Oh Is A Common Automobile Antifreeze Calculate The Freezing Point And Boiling Point Of Homeworklib
Ethylene Glycol Molecule Of The Month June 2018 Html Version
Solved Ethylene Glycol Is Used As An Automobile Antifreeze And In The Manufacture Of Polyester Fibers The Name Glycol Stems From The Sweet Taste Of This Poisonous Compound Combustion Of 6 38 Mathrm Mg Of

2 An Automobile Mechanic Mixes Ethylene Glycol With Water To Make A Solution Of Automobile Antifreeze Homeworklib

Solved Ethylene Glycol Is Used As Antifreeze In Automobile Chegg Com
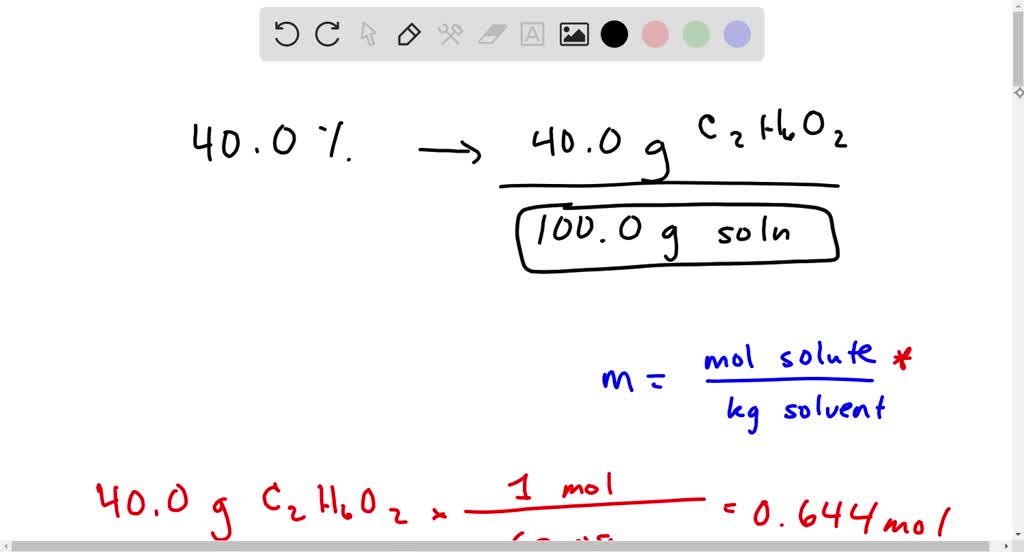
Solved What Is The Molality Of The 40 0 Mass Ethylene Glycol Solution Used For Automobile Antifreeze Problem 11 61

Solved Ethylene Glycol Is Used As An Automobile Antifreeze And In The Manufacture Of Polyester Fibers The Name Glycol Stems From The Sweet Taste Of This Poisonous Compound Combustion Of 6 38 Mathrm Mg Of

Amazon Com Dial Type Quick Test Antifreeze Density Meter Ethylene Glycol Coolant Automobile Antifreeze Tester Coolant Tester Accessories Automotive

Ethylene Glycol Is Used As Coolant In Car Radiators In Order To Prevent The Solution From Freezing At 0 3 O C The Amount Of Ethylene Glycol To Be Added To 5

Buy Antifreeze Refractometer 3 In 1 Coolant Tester For Checking Freezing Point Concentration Of Ethylene Glycol Or Propylene Glycol Based Automobile Antifreeze Coolant And Battery Acid Condition Online In Indonesia B07xcnccym

Comments
Post a Comment